Myeloproliferative neoplasms (MPNs) are cancers that arise when a mutated blood stem cell begins to produce too many mature blood cells. A number of mutations can drive MPNs, and studies have demonstrated that different mutations result in different clinical outcomes. Last year, for example, former Damon Runyon Clinical Investigator Ann L. Mullally, MD, and her lab at Dana-Farber Cancer Institute found that patients with mutations in the JAK2 gene responded far better to immunotherapy than did patients with mutations in the CALR gene.
Since then, the team has sought to address the lack of targeted therapies for CALR-mutated MPNs, which account for about a quarter of all cases. In a recent study, Dr. Mullally and her colleagues screened the entire genome of CALR-mutant blood stem cells to identify their unique genetic features—i.e., potential therapeutic targets. Using the gene editing tool CRISPR, the team systematically knocked out every gene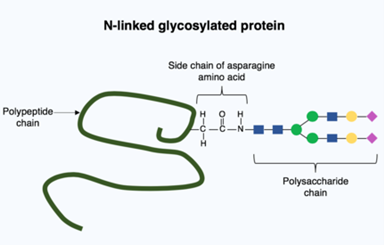 that might play a role in driving cancer progression in these cells.
that might play a role in driving cancer progression in these cells.
According to their results, the growth of CALR-mutant cells depends heavily on a process called N-glycosylation, by which sugar molecules are attached to the nitrogen atoms in proteins. The researchers tested this dependency by chemically inhibiting the N-glycosylation pathway, and indeed they were able to impair the growth of CALR-mutant cells and reduce features of MPN in mouse models.
With this study, Dr. Mullally and her team have exposed genetic vulnerabilities of CALR-mutated blood stem cells and demonstrated how to exploit their therapeutic potential. In other words, having established the importance of mutation-specific care in MPNs, the Mullally Lab is now showing us what that care might look like.
This research was published in Blood.







