‘‘All happy families are alike; each unhappy family is unhappy in its own way.’’ This principle, borrowed from Leo Tolstoy, is how Damon Runyon alumni Pavan Bachireddy, MD, and Catherine J. Wu, MD, summarized the conditions of immunotherapy response and resistance in a recent study.
Immunotherapies activate the body’s own immune T cells to seek and destroy cancer cells. These therapies are remarkably effective for a subset of cancer patients but less so for others—and the factors that determine whether immunotherapy will be helpful or not are not well understood.
To better understand how T cells differ between patients who benefit from immunotherapy and those who do not, the team collected bone marrow samples from twelve leukemia patients before and after they received an immunotherapy known as donor lymphocyte infusion (DLI). Six experienced complete remission, while the other six saw no measurable reduction in tumor size. The team sequenced a total of nearly 400,000 T cells across the cohort to examine how each group’s T cell population evolved differently over the course of treatment.
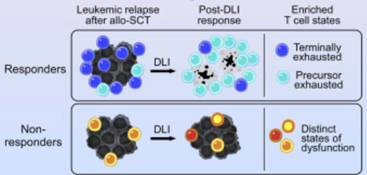 Like all cells, T cells arise from a process called differentiation, whereby they develop from cells with the potential to serve multiple functions into immune cells with a specialized function. In this study, the researchers found that all six responsive patients had something in common: a concentration of late-differentiated (i.e., terminal) T cells prior to treatment, and rapid expansion of early-differentiated (i.e., precursor) T cells following treatment. In patients whose tumors resisted immunotherapy, however, they identified multiple types of T cell dysfunction. In other words, to paraphrase Tolstoy, immunotherapy response tends to follow a pattern, while resistance looks different from patient to patient.
Like all cells, T cells arise from a process called differentiation, whereby they develop from cells with the potential to serve multiple functions into immune cells with a specialized function. In this study, the researchers found that all six responsive patients had something in common: a concentration of late-differentiated (i.e., terminal) T cells prior to treatment, and rapid expansion of early-differentiated (i.e., precursor) T cells following treatment. In patients whose tumors resisted immunotherapy, however, they identified multiple types of T cell dysfunction. In other words, to paraphrase Tolstoy, immunotherapy response tends to follow a pattern, while resistance looks different from patient to patient.
The secret to a “happy” treatment outcome was further illuminated when the team investigated a follow-up question: where does this post-treatment influx of precursor T cells come from? Not, as one might have guessed, from the donor infusion, but from the patient’s own precursor T cell population. This suggests that DLI works not by directly introducing new immune cells, but by helping to recruit the body’s pre-existing immune cells to the tumor environment.
Given Dr. Bachireddy and Dr. Wu’s findings, future clinical trials might consider using patients’ late-differentiated T cell count as a means of predicting DLI response. They might also evaluate therapeutic strategies that enhance precursor T cell recruitment, given what we now know about how DLI works. Hopefully, with further research, immunotherapy drugs will benefit cancer patients in ways more alike than different.







