New approach uncovers epigenetic drivers of pediatric leukemia
While some cancers are known to be caused by mutations in key genes, genetic mutation does not always tell the full story. Epigenetic changes—which do not affect the DNA sequence itself, but rather the degree to which a gene is expressed—can play an important role in cancer as well. Such is the case with acute lymphoblastic leukemia (ALL), the most common form of cancer in children, which has a low incidence of genetic mutation but often coincides with abnormal epigenetic behavior.
The epigenetic mechanism that switches genes “off” is called methylation, as it involves attaching a methyl (CH3) group to the DNA. Previous studies have suggested that ALL is driven by irregularities in DNA methylation, but because these investigations have focused only on a few regions of the genome, their applications in the clinic have been limited. With a novel probability-based approach, however, former Sohn Fellow Michael Koldobskiy, MD, PhD, and colleagues at Johns Hopkins have been able to analyze methylation patterns across the entire genome of pediatric ALL patients, generating remarkable insight into the epigenetic drivers of this disease.
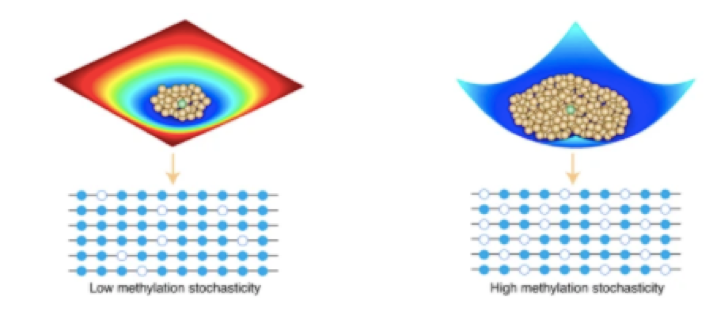
stochasticity (randomness) in normal and cancer cells.
The team’s whole-genome analysis revealed increased entropy, or randomness, in the methylation of specific regions of the genome, including known ALL-associated genes. (How is randomness measured, one might ask? Using information theory, or the measure of how much information is contained within one data point—a novel element of Dr. Koldobskiy’s approach.) Notably, the results show that even non-mutated genes are subject to this epigenetic instability. These findings suggest a network of genetic and epigenetic factors underlying ALL, which may explain the genetic diversity of pediatric ALL patients, and illuminate future therapeutic avenues.
Research has shown that epigenetic changes underlie many types of cancer. Increased randomness in methylation, as seen in ALL, is thought to drive cancer by selecting for cells that are most able to survive and grow in a changing environment. Furthermore, studies of other blood cancers have found that methylation patterns may have prognostic import (i.e., a higher degree of randomness correlates with a worse clinical outcome). As researchers increasingly recognize the importance of epigenetic regulation in cancer, investigations like those led by Dr. Koldobskiy offer clues not just to ALL but to broader unanswered questions in cancer pathology.
Learn more about this research in Nature Biomedical Engineering.
