Just as the study of a growing plant or animal must take into account its environment, cancer researchers must look beyond a tumor to understand how the surrounding tissue impacts its development. In the case of gliomas, the most common and aggressive type of brain tumor, this means looking at neurons—what signals they emit, and how these signals may play a role in brain tumor progression.
A recent study by former Damon Runyon-Sohn Fellow Kathryn R. Taylor, PhD, and her colleagues at Stanford University sheds new light on these questions.
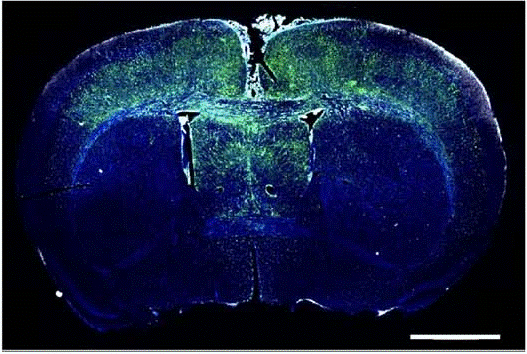
In healthy brain tissue, neurons release growth factors that promote neuroplasticity, or the ability of brain cells to change and create new neural networks—what, in regular life, we call “learning.” But as Dr. Taylor and her team discovered, once these growth factors are released, their effect may be broader than intended. Glioma cells, they observed, have receptors for the same growth factors and exhibit a similar plasticity as normal brain cells, suggesting that they benefit from the same signaling pathways that promote healthy brain activity. In other words, what enables memory formation and learning may also give rise to “smarter,” more adaptable tumors.
The good news is that Dr. Taylor and her colleagues identified a specific growth factor, known as brain-derived neurotrophic factor (BDNF), that kicks off the signaling pathway. By blocking this signal, either genetically or with a drug molecule, the team was able to prolong survival in mouse models of pediatric and adult gliomas. These findings suggest new therapeutic potential for BDNF pathway inhibitors.
“Gliomas hijack processes of neural plasticity, leveraging mechanisms that normally […] contribute to cognition in the healthy brain,” Dr. Taylor explains. “Understanding and targeting these mechanisms in glioma may be critical for the effective treatment of these deadly brain cancers.”
This research was published in Nature.







